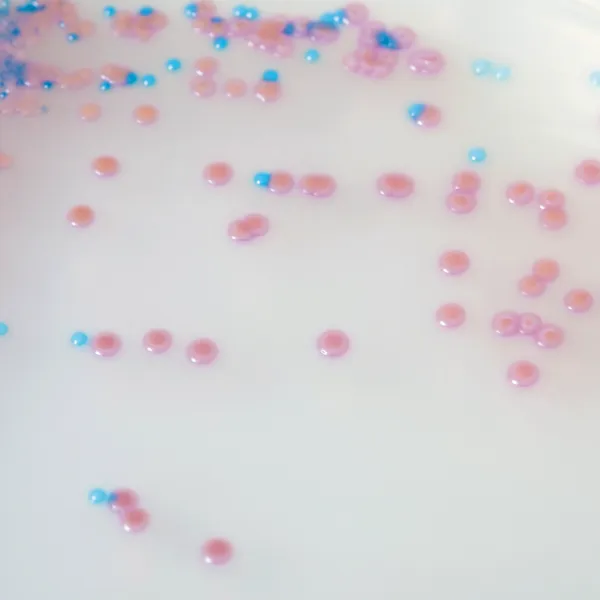
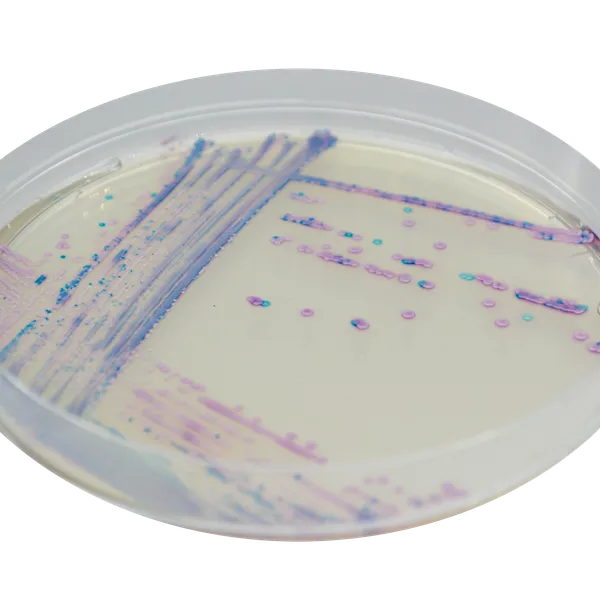
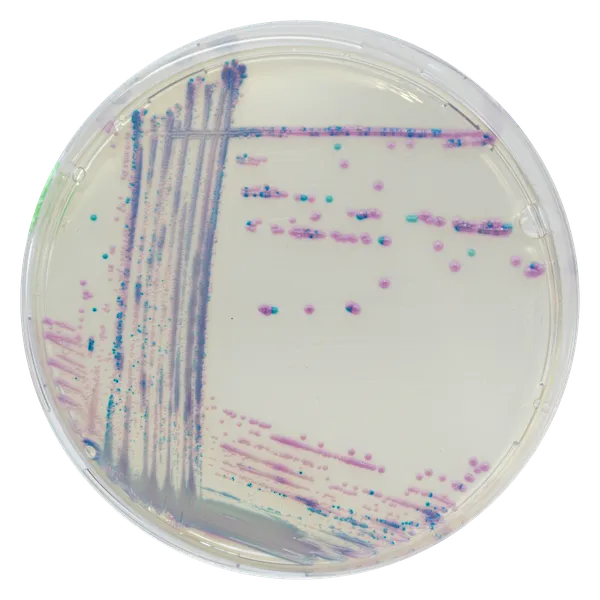
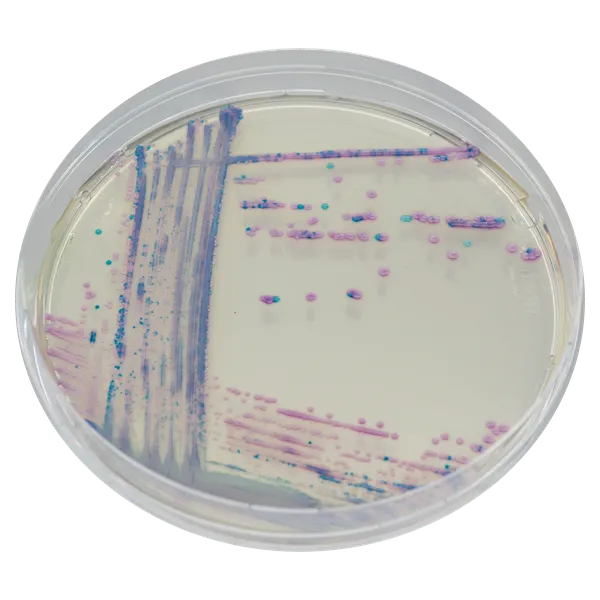
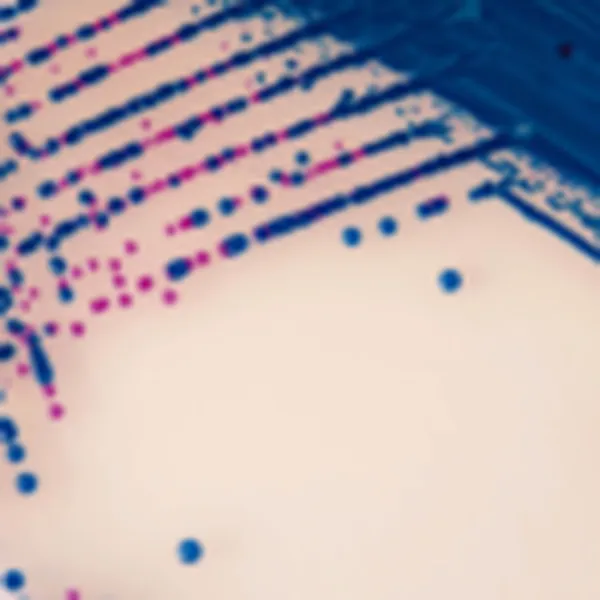
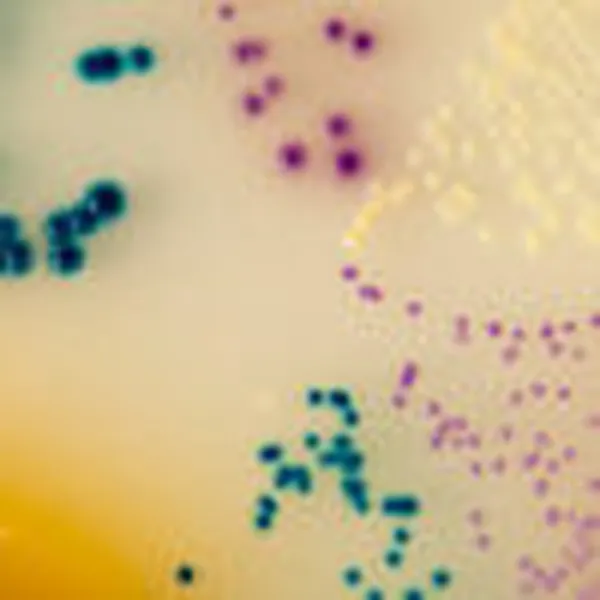
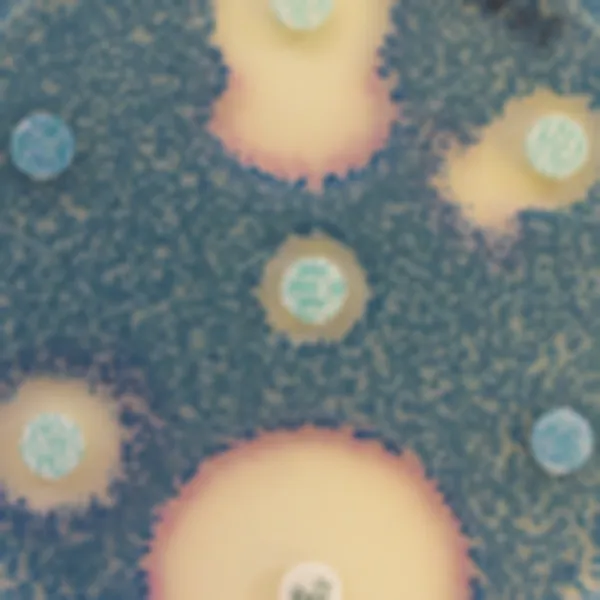
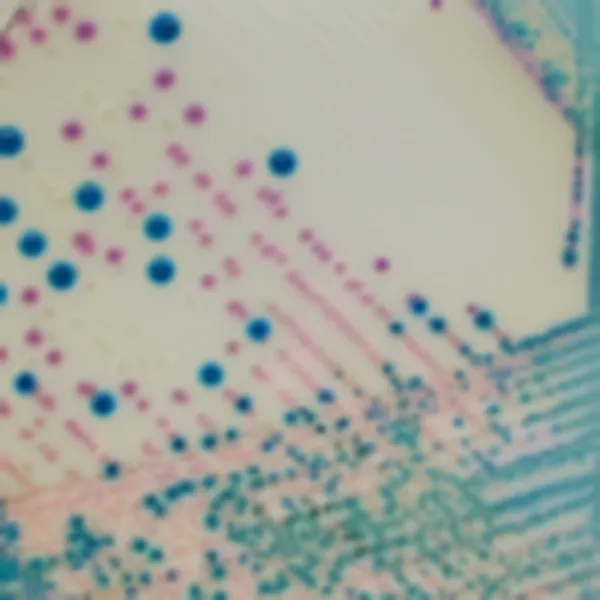
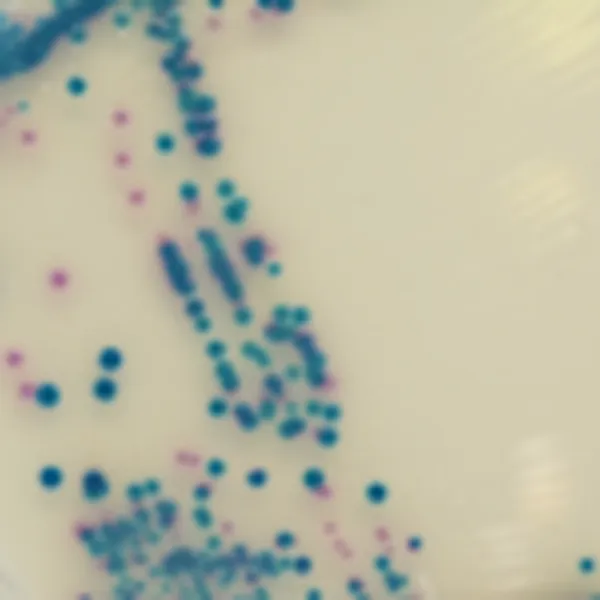
Colonies Appearance

S. aureus
pink to mauve

Other bacteria
colourless, blue or inhibited
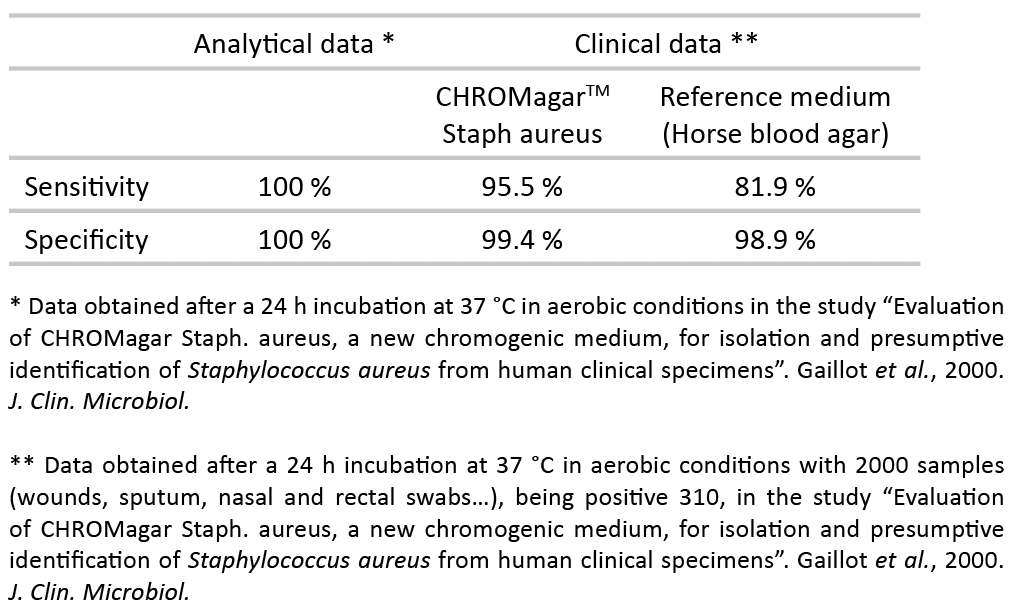
Performance
Performance
Intended Use :
CHROMagar™ Staph aureus is a selective chromogenic culture medium intended for use in the qualitative direct detection, differentiation and presumptive identification of Staphylococcus aureus to aid in the diagnosis of S. aureus colonization. The test is performed with swabs from teguments, wounds or soft tissue specimens. Results can be interpreted after 18-24 h of aerobic incubation at 35-37 °C.
The medium can also be used as an early warning indicator for diagnostic tests of infections to signal the possible presence of S. aureus. This use does not replace the institution’s protocols.
Concomitant cultures are necessary to recover organisms for further microbiological testing or epidemiological typing. A lack of growth or the absence of colonies on CHROMagar™ Staph aureus does not preclude the presence of S. aureus. CHROMagar™ Staph aureus is not intended to diagnose infection nor to guide nor monitor treatment for infections.
CHROMagar™ Staph aureus can also be used in the detection of S. aureus in the analyses of food products for human consumption, animal feed and in environmental samples.
Food & environmental quality control:
Human beings are the main reservoir of S. aureus. A carrier contaminates the surrounding environment when coughing, sneezing and by touching food with a hand. It is often found in the environment and on food preparation surfaces and also in certain uncooked foods (dairy products, salads, sandwiches...). It is important to check the presence of S. aureus before and after the foodstuff sterilisation process.
1. Easy to prepare: The conventional medium for S. aureus is the Baird-Parker which has to be supplemented with RPF (Rabbit Plasma Fibrinogen), rendering the plate manufacturing delicate and complex, and also reducing the shelf life of the poured plates to a couple of weeks.
On the contrary, CHROMagar™ Staph aureus comes with all the compounds already in the agar (no need of any supplement) and remains stable.
2. Fast: The results on Baird Parker have to be read after 48 h of incubation while with CHROMagar™ Staph aureus, the results are available after only 24 h.
Clinical application :
S. aureus is the leading cause of skin and soft tissue infections and can also cause serious infections such as bloodstream infections, pneumonia, or bone and joint infections.
1. Easy to read compared to Blood agar or Mannitol Salt Agar. CHROMagar™ Staph aureus allows easier differentiation of S. aureus colonies enhanced by a mauve colour and is of considerable help in identifying suspect colonies. thus, it reduces the confirmatory workload.
Composition
Technical Documents
Scientific Publications
2023
CHROMagar Staph aureus for the enumeration at 37°C of Staphylococcus aureus and other coagulase positive staphylococci in human food and animal feed products
📄 Publication2018
Validation and implementation of Colorex™ CHROMagar™ Staph aureus on WASP/WASPLab™ for screening for Staphylococcus aureus using the Eswab™
📄 Publication2018
Multicentre Validation of a Chromogenic Medium for Screening of Staphylococcus aureus in Respiratory Samples from Cystic Fibrosis Patients
📄 Publication2004
Optimal detection of Staphylococcus aureus from clinical specimens using a new chromogenic medium
📄 Publication2003
Use of a new chromogenic culture medium facilitates detection of Staphylococcus aureus in a screening program
📄 Publication2001
Performance of the chromogenic medium CHROMagar Staph aureus and the Staphylochrom coagulase test in the detection and identification of Staphylococcus aureus in clinical specimens
📄 Publication2012
Performance of CHROMagar Staph aureus and CHROMagar MRSA for Detection of Airborne Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus DOI: 10.1080/02786826.2011.626001
📄 Publication2011
Evaluation of CHROMagar and Pastorex Test in Identification of Staphylococcus aureus Afaf Abd El Rahman, Abeer El sayed and Afaf Mahmood
📄 Publication2004
Evaluacion del nuevo medio cromogenico “CHROMagar Staph aureus” para identification presuntiva de S.aureus (Poster in spanish)
📄 Publication2003
Evaluation of Chromogenic Medium for the Rapid and Presumptive Identification of Staphylococcus aureus from Food Specimens
📄 Publication2001
Evaluation of CHROMagar Staph aureus, a new chromogenic medium, for isolation and presumptive identification of Staphylococcus aureus from human clinical specimens
📄 Publication2000
Dépistage nasal de Staphylococcus aureus. Nécessité de standardiser les protocoles
📄 Publication














Read more